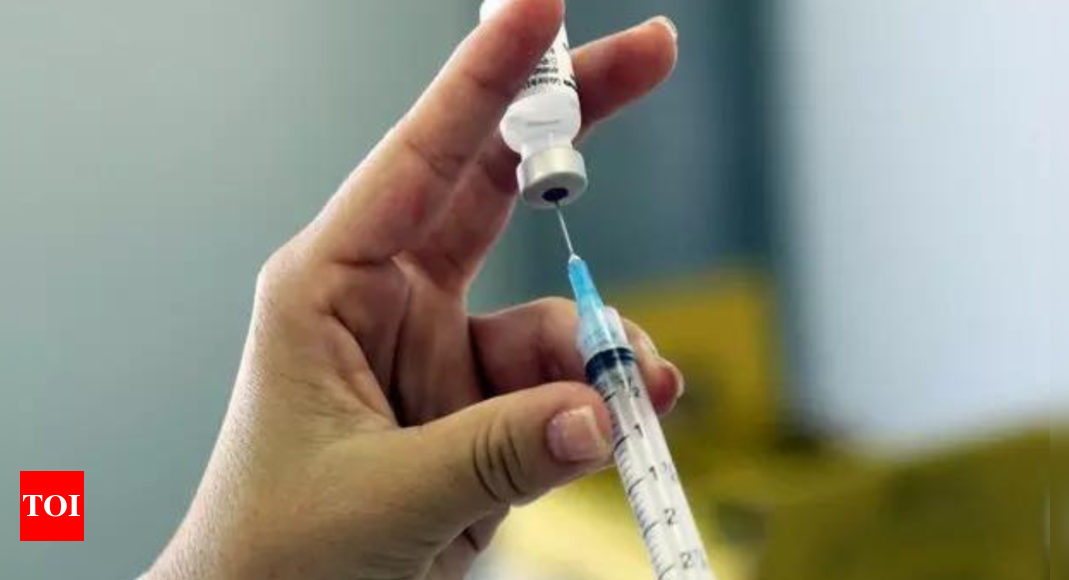
HYDERABAD: Japanese pharma giant Takeda has entered into a strategic partnership with Hyderabad-based biopharma player Biological E Limited (BE), to manufacture up to 50 million doses of Takeda’s dengue vaccine QDENGA per annum.
Takeda said this tie-up will accelerate its ability to deliver 100 million doses of the dengue vaccine by 2030.
As part of the partnership, BE will ramp up its manufacturing capacity to up to 50 million doses a year.
The alliance was announced at the inaugural session of BioAsia 2024 lifesciences conclave here on Tuesday.
“Takeda’s long-term goal for our dengue program has been to make QDENGA broadly available to those at risk who may benefit from immunization. Within the last year, we’ve successfully launched in private markets, are now launching in some public programs, and working with partners to support a broader public health impact,” said Gary Dubin, president-Global Vaccine Business Unit, Takeda.
“We are proud to announce a strategic manufacturing partnership with Biological E. Limited, which has deep expertise in vaccine manufacturing and longstanding support of public health programs around the world. Together, we will help combat dengue on a global scale by significantly increasing manufacturing capacity for multi-dose vials of QDENGA to drive sustainable access to the vaccine in more endemic countries,” he said.
BE managing director Mahima Datla said: “We are proud to collaborate with Takeda in the production of their groundbreaking Dengue Tetravalent Vaccine, QDENGA, in multi-dose vials. Takeda’s commitment to patient-focused, value-based research and development aligns extremely well with our dedication to advancing healthcare.”
“We are fortunate to have created an institute that attracts such strong global partners for complex vaccines and underscores our shared mission of shaping a healthier future for all. With Takeda’s esteemed history and global presence, we are honoured to advance our vision of delivering highly innovative medicines and transformative care worldwide,” she added.
QDENGA is a live attenuated dengue tetravalent vaccine that comes in multi-dose vials. QDENGA is currently available for children and adults in the private market in countries in Europe as well as Indonesia and Thailand, and in private and some public programs in Argentina and Brazil. TAK-003 is not approved for use in India.
Takeda said this tie-up will accelerate its ability to deliver 100 million doses of the dengue vaccine by 2030.
As part of the partnership, BE will ramp up its manufacturing capacity to up to 50 million doses a year.
The alliance was announced at the inaugural session of BioAsia 2024 lifesciences conclave here on Tuesday.
“Takeda’s long-term goal for our dengue program has been to make QDENGA broadly available to those at risk who may benefit from immunization. Within the last year, we’ve successfully launched in private markets, are now launching in some public programs, and working with partners to support a broader public health impact,” said Gary Dubin, president-Global Vaccine Business Unit, Takeda.
“We are proud to announce a strategic manufacturing partnership with Biological E. Limited, which has deep expertise in vaccine manufacturing and longstanding support of public health programs around the world. Together, we will help combat dengue on a global scale by significantly increasing manufacturing capacity for multi-dose vials of QDENGA to drive sustainable access to the vaccine in more endemic countries,” he said.
BE managing director Mahima Datla said: “We are proud to collaborate with Takeda in the production of their groundbreaking Dengue Tetravalent Vaccine, QDENGA, in multi-dose vials. Takeda’s commitment to patient-focused, value-based research and development aligns extremely well with our dedication to advancing healthcare.”
“We are fortunate to have created an institute that attracts such strong global partners for complex vaccines and underscores our shared mission of shaping a healthier future for all. With Takeda’s esteemed history and global presence, we are honoured to advance our vision of delivering highly innovative medicines and transformative care worldwide,” she added.
QDENGA is a live attenuated dengue tetravalent vaccine that comes in multi-dose vials. QDENGA is currently available for children and adults in the private market in countries in Europe as well as Indonesia and Thailand, and in private and some public programs in Argentina and Brazil. TAK-003 is not approved for use in India.